Soil Science Spotlight: One of the primary functions of the GROW BIOINTENSIVE method is to allow small-scale farmers everywhere to build and maintain soil fertility levels that will allow the farmers to grow a large amount of food and compost materials in a very small area, with greatly reduced resource use, for an indefinite period of time, sustainably. Soil testing and the application of the correct type and quantity of organic soil amendments at the correct time is a fundamental part of building and maintaining sustainable soil fertility levels. To introduce the topic of soil testing and the reasoning and methodology involved in soil test analysis and making soil amendment recommendations to a wider audience, John Beeby and Ecology Action are creating a series of topics on the subject called “Soil Science Spotlight”, which is posted to growbiointensive.org in the “Protocol” section with new posts added often.
In parts 1-3 of this segment, I introduced Dr. John
Doran's USDA Soil Quality Test Kit Guide Soil pH has not always been recognized as an important parameter for soil health. Even the famed soil scientist Dr. William Albrecht discounted the importance of soil pH. However, we have learned a lot about soils and crops over the last 50 to 60 years! Soil pH has been shown repeatedly to be a fundamental property of soils that affects nutrient availability and the health of macro- and microorganisms. In addition, because all parts of soils are connected, soil pH can affect soil structure and the ability of air and water to enter the soil, and can also affect the nutrient-holding capacity of organic matter and some clays in the soil.
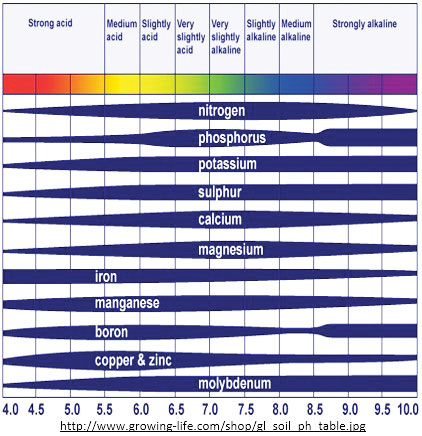
We can see in the graphic (above) that a nutrient might be present in the soil, but not available for the crops to take up simply because of the soil’s pH. The width of the dark blue region for each nutrient represents its availability to crops at each of the pHs shown below; the wider the area, the more available it is. So what is pH and why does it have such an effect on soil nutrient availability? pH is simply a measure of the amount of hydrogen ions (H+) in the soil. The more hydrogen atoms, the lower the soil pH and the more acidic the soil is. The opposite ion, the yin to hydrogen’s yang, is hydroxide (OH-). The more hydrogen there is, the less hydroxide there is and vice versa, so the more hydroxide is present, the higher the soil pH and the more alkaline the soil is. So what does all this mean for nutrient availability? Nutrients are largely taken up by plants as ions. Most agricultural soils have a pH between 5 and 8. We can see from the graphic below that many agriculturally important nutrients, such as nitrogen, potassium, sulfur (sulfate), calcium, and magnesium, are readily available in this pH range. However, some nutrients, like phosphorus—of which crops need large quantities—are only readily available in the range between pH 6 and 8. This is entirely due to the nature of phosphate (PO₄3-) and specifically its very large negative charge (3-). When the soil’s pH is less than 6, soils typically have a lot of free iron and free aluminum, both of which have a high positive charge: Iron (Fe3+) and aluminum (Al3+). These highly positively charged ions are naturally attracted to highly negatively charged phosphate, and so they bind with phosphate through ionic bonds to form either iron or aluminum phosphate. This binding process, called precipitation, causes these free ions to become minerals and no longer available for crop root uptake. Then, when the pH is 8 or above, typically soils have a lot of free calcium (Ca2+) which is also highly attracted to phosphorus due to its highly negative charge. There are additional chemical complexities involved that explain phosphorus’s availability above 8.5, but since most agricultural soils are not this alkaline, we will skip over more chemistry for now. The other set of nutrients whose availability is highly dependent on soil pH are the trace elements or minor elements, namely iron, manganese, boron, copper, and zinc (as well as molybdenum). These are readily available in acidic soils, but much less available in alkaline soils. Using iron as an example: when a soil becomes alkaline, it has a lot of hydroxide ions (which are negatively charged) and very few hydrogen atoms, as mentioned before. The abundant negatively charged hydroxide ions react with iron (as well as zinc, manganese, and copper) to form iron oxide. This transformation causes iron ions to become minerals, and unavailable for crop root uptake. The abundance of hydroxide ions also reduces boron availability, but in this case it is not ionic bonding that occurs, but a more complex interaction called ligand exchange. Okay, so that was a lot of chemistry to explain why the availability of some nutrients (most phosphorus and the trace elements) is highly dependent on pH. But what does it mean? It means that if your crops are showing consistent symptoms of iron deficiency (for example), it is quite possible that your soil has plenty of iron in it, but it is just not available in sufficient quantities in ionic (plantavailable) form to meet the needs of your crops. If you know iron availability is pH-dependent, rather than wasting money on iron sulfate fertilizer (which won't help your plants if pH is causing the iron to be unavailable), you could consider first testing your soil’s pH, and then, assuming the test shows the soil is too alkaline, lowering it with elemental sulfur, thus making the iron already present in your soil more available and able to meet your crops’ needs. Soil pH also plays an important role in ensuring the soil is a healthy environment that can support a diversity of soil macro- and microorganisms. Like crops, most soil life thrives in a soil environment that is between a pH of 6 and 7. If the soil pH is outside of that range, it will only be able to support a limited number of species that have adapted to that harsher environment. Of course, soil life also needs sufficient air, water, organic matter, and nutrients to thrive, and not just a specific pH range, but just like with plants, pH affects nutrient availability for many microbes. A happy, healthy, diverse population of soil life that has plenty of air, water, organic matter, and nutrients can naturally create healthy soil with good soil structure, allowing air and water to pass easily into soils and allowing soils to hold onto more water and produce good crops even under drought conditions. Soil pH also affects the ability of a soil’s organic matter, 1:1 clays, and oxides contained with the soil, to hold onto nutrients needed by crops and prevent those important nutrients from leaching and being lost. If you are blessed with a soil that has a lot of 2:1 clay in it, your soil has a strong ability to hold onto nutrients regardless of the soil’s pH. However, the other compounds in soil that are able to hold onto nutrients, namely 1:1 clays, oxides, and organic matter, all have reduced abilities to hold onto nutrients when the soil pH is low (acidic soils). While adding compost to an acidic soil will help that soil in a number of ways, including improved soil structure, the power of that compost to hold nutrients is greatly reduced by the soil’s low pH. So, it is important to keep in mind that in order to improve the fertility and long-term productivity of an acidic soil, you must not only add compost but also increase the pH of that soil by adding lime. Then, that soil can retain more of the nutrients it naturally has, and can retain more nutrients that you may add through fertilization once you understand what nutrients the soil needs and does not need to increase its fertility. ♥ top | Newsletter Home |Table of Contents| Archive
|